Chemistry Form 4 Chapter 7 Acid and Base
If section 46 were moved to chapter 12 then 56 and 75 would likely need to be moved into an organic or biological chemistry chapter as well. 12-Dihydroxylation the conversion of the CC double bond to 12-diol is an oxidative addition reaction of alkene.

Pin By Sanghita Dey On Cbse Class 10 Concentration Activities Acids Bases And Salts Equations
The differences listed above depicts the clear difference between acids and bases which forms part of the chemistry and discussed among students the world over.

. Write chemical reaction of aniline with. Acid Base And Salt. Write reactions of the final alkylation product of aniline with excess of methyl iodide in the presence of sodium carbonate solution.
In chemistry an acid dissociation constant also known as acidity constant or acid-ionization constant. Air for example is a solution. Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other.
Complete the following acid-base reactions and name the products. The NCERT Solutions Class 7 Science Chapter 5 Acids Bases and Salts is crucial due to the applications it has in science and the impact it puts on your future studies as well as on CBSE Class 7 examinations. NCERT Solutions for Class 7 Science Chapter 5 Acids Bases and Salts.
This is the base of Chemistry and what must be noted is that a lot of the important objectives - easy marks questions form the base of this chapter appear every year in Class 11 Chemistry Exam. Denoted is a quantitative measure of the strength of an acid in solutionIt is the equilibrium constant for a chemical reaction known as dissociation in the context of acidbase reactionsThe chemical species HA is an acid that dissociates into A the conjugate base of. Solutions are all around us.
I CH 3 CH 2 CH 2 NH 2 HCl ii C 2 H 5 3 NHCl Ans. Because each formula unit of NH 4 2 Cr 2 O 7 produces three ions when dissolved in water 2NH 4 1Cr 2 O 7 2 the total concentration of ions in the solution is 3 143 M 429 M. Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more.
Acidity is indicated by a pH less than 7 while a pH greater than 7 indicates a base. Potassium permanganate can be used as well though further oxidation is prone to occur to cleave the diol because it is a stronger oxidizing agent 1072. Modification of work.
If you live near a lake a river or an ocean that body of water is not pure H 2 O but most probably a solution. The equivalent value of NH 4 2 Cr 2 O 7 can then be calculated by dividing 143 M by 429 M. Modification of work by the Italian voiceFlickr.
The NCERT Solutions provided here are. The other sections that could fit within either a general or organicbiological chemistry chapter are sections 56 redox in organic and biochemistry and 75 energy of biochemical reactions. PH is a measurement of the proportion of free hydrogen and hydroxyl ions in water.
Osmium tetroxide OsO 4 is a widely used oxidizing agent for such purpose. Thus these notes are essential to refer before attempting. Class 11th Chemistry Chapter 1 notes cover all these crucial questions with solved problems.
Modification of work by vxlaFlickr.

Determining Ph Of A Solution Acidic Basic Neutral Solutions Video Lesson Transcript Study Com
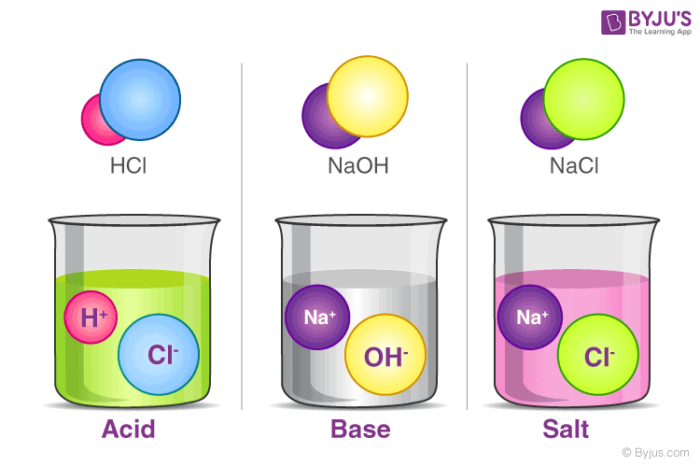
Acids Bases And Salts Definition Dissociation Neutralization Of Acids Bases And Salts
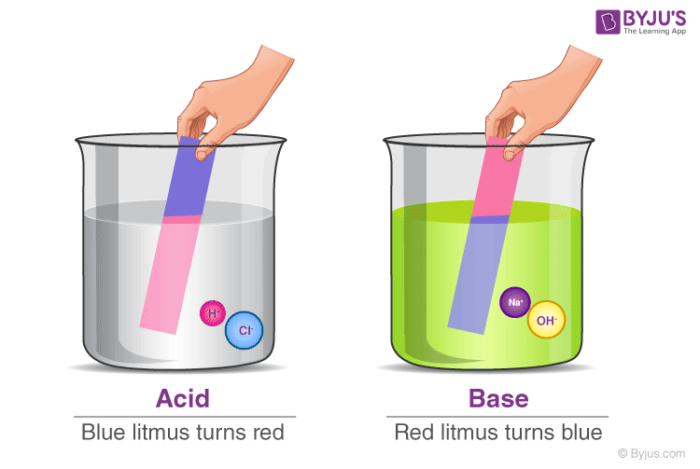
Difference Between Acid And Base Differences Btw Acid Base In Tabular Form



I found your blog post on Chemistry Form 4 Chapter 7 - Acid and Base incredibly helpful. The way you've explained the concepts and provided examples is very clear and makes it easier to understand this challenging topic. As a student studying chemistry, this resource is a great aid for me. Concentration Exercises For Students
ReplyDeleteThank you for sharing your knowledge and making learning more accessible!